By: Rajini KG
August 3 2023
No, video doesn't show assault on Hindu priest after being 'caught' with two women
The Verdict False
The video is of an attack on a Buddhist monk and two women in Sri Lanka. It has also been falsely linked to a deceased self-styled spiritual leader.
Context
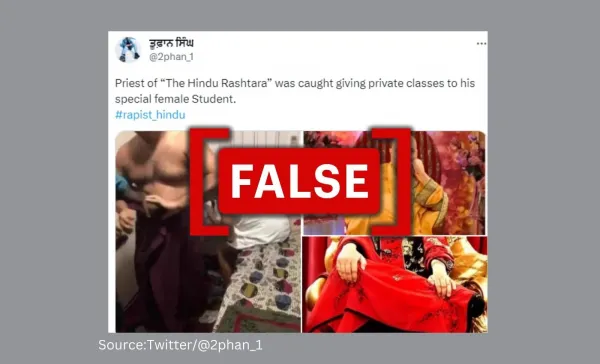
A video of some people attacking a man and two women in a room is circulating online, with claims insinuating that it purportedly shows a Hindu priest who was "caught" with two women. Some posts have also been shared on Twitter(now called X) and Facebook with screenshots from the video and images of the late self-styled spiritual leader Nirmal Singh Maharaj, popularly known as Guruji, implying that it was him in the video. One such post read: "Another Baba in the field after #Asharam and #RamRahim The population of #blind devotees is not increasing just like this, many Baba ji are working hard Congratulations #अंधभक्तो." The archive of the posts can be found here and here.
However, the man in the viral video is neither a Hindu priest nor Nirmal Singh Maharaj.
In fact
A reverse image search of the keyframes from the viral video led us to local news reports from Sri Lanka. A news report by Asian Mirror, a Sri Lankan news outlet, published on July 8, carried a still from the viral video. According to the report, four people attacked one Pallegama Sumana Thero and two women in a residential house in Navagamuwa, in the Rassapana administrative area of Sri Lanka. Following the attack, a Theroa filed a complaint at the Navagamuwa police station. The assailants reportedly claimed that they had received information that Theroa was allegedly involved in an illicit relationship with two women and that they assaulted him based on that information. Later, Thero withdrew the police complaint, and the assailants were released on bail. The report referred to Theroa as a monk and noted that he held a prominent position in the community.
In a report on the incident, the multi-lingual ETV Bharat news website referred to Thero as a Buddhist monk. The Sinhalese report added that the Sri Lankan government had said that legal action would be taken against the people spreading the video with Thero and the two women on social media.
According to a report by BBC News Sinhala, Thero initially worked in Ganewatta Purana Vihara (a Buddhist temple) for about 20 years. Later, he built a temple called Sri Sumanaramaya temple (also a Buddhist temple) in Wakewatta and lived there, the report, which included a photo of Thero, added.
These news reports prove that the viral video is from Sri Lanka, and the man in it is Pallegama Sumana Thero, a Buddhist monk and not a Hindu priest. The video has also been falsely linked to the late Nirmal Singh Maharaj, who passed away in 2007.
The verdict
A video of a Buddhist monk from Sri Lanka, and an image of the late self-styled spiritual leader Nirmal Singh Maharaj, have been wrongly linked. Therefore, we mark the claim false.


